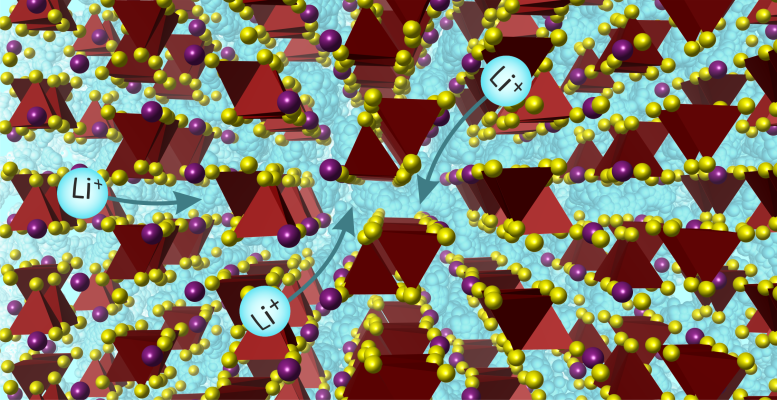
A team from the University of Liverpool has developed a new solid lithium-ion conductor that could replace liquid electrolytes in batteries, enhancing safety and efficiency. This discovery, facilitated by AI and interdisciplinary collaboration, paves the way for further advancements in sustainable energy storage solutions. Image represents the lithium ions (in blue) moving through the structure. Credit: University of Liverpool
Researchers at the University of Liverpool have identified a novel solid substance capable of swiftly conducting lithium ions.
A major challenge in the field of materials science involves developing and identifying novel materials that contribute towards achieving global objectives, including the pursuit of Net Zero.
In a paper published in the journal Science, researchers at the University of Liverpool have discovered a solid material that rapidly conducts lithium ions. Such lithium electrolytes are essential components in the rechargeable batteries that power electric vehicles and many electronic devices.
Consisting of non-toxic earth-abundant elements, the new material has high enough Li ion conductivity to replace the liquid electrolytes in current Li ion battery technology, improving safety and energy capacity.
Using a transformative scientific approach to design the material, the interdisciplinary research team from the University synthesised the material in the laboratory, determined its structure (the arrangement of the atoms in space), and demonstrated it in a battery cell.
The Role of AI and Collaborative Research
The new material is one of a very small number of solid materials that achieve Li ion conductivity high enough to replace liquid electrolytes, and operates in a new way because of its structure.
Its discovery was achieved through a collaborative computational and experimental workflow that used AI and physics-based calculations to support decisions made by chemistry experts at the University.
The new material provides a platform for the optimization of chemistry to further enhance the properties of the material itself, and to identify other materials based on the new understanding provided by the study.
Impact and Future Directions
Professor Matt Rosseinsky, from the University of Liverpool’s Department of Chemistry, said: “This research demonstrates the design and discovery of a material that is both new and functional. The structure of this material changes the previous understanding of what a high-performance solid-state electrolyte looks like.
“Specifically, solids with many different environments for the mobile ions can perform very well, not just the small number of solids where there is a very narrow range of ionic environments. This dramatically opens up the chemical space available for further discoveries.
Recent reports and media coverage herald the use of AI tools to find potentially new materials. In these cases, the AI tools are working independently and thus are likely to recreate what they were trained on in various ways, generating materials that may be very similar to known ones.
“This discovery research paper shows that AI and computers marshaled by experts can tackle the complex problem of real-world materials discovery, where we seek meaningful differences in composition and structure whose impact on properties is assessed based on understanding.”
“Our disruptive design approach offers a new route to the discovery of these and other high-performance materials that rely on the fast motion of ions in solids.”
Reference: “Superionic lithium transport via multiple coordination environments defined by two-anion packing” by Guopeng Han, Andrij Vasylenko, Luke M. Daniels, Chris M. Collins, Lucia Corti, Ruiyong Chen, Hongjun Niu, Troy D. Manning, Dmytro Antypov, Matthew S. Dyer, Jungwoo Lim, Marco Zanella, Manel Sonni, Mounib Bahri, Hongil Jo, Yun Dang, Craig M. Robertson, Frédéric Blanc, Laurence J. Hardwick, Nigel D. Browning, John B. Claridge and Matthew J. Rosseinsky, 15 February 2024, Science.
DOI: 10.1126/science.adh5115
The study undertaken was a combined effort between researchers in University of Liverpool’s Department of Chemistry, Materials Innovation Factory, Leverhulme Research Centre for Functional Materials Design, Stephenson Institute for Renewable Energy, Albert Crewe Centre, and School of Engineering.
The work was funded by the Engineering and Physical Sciences Research Council (EPSRC), the Leverhulme Trust, and the Faraday Institution.


